Download our whitepaper,
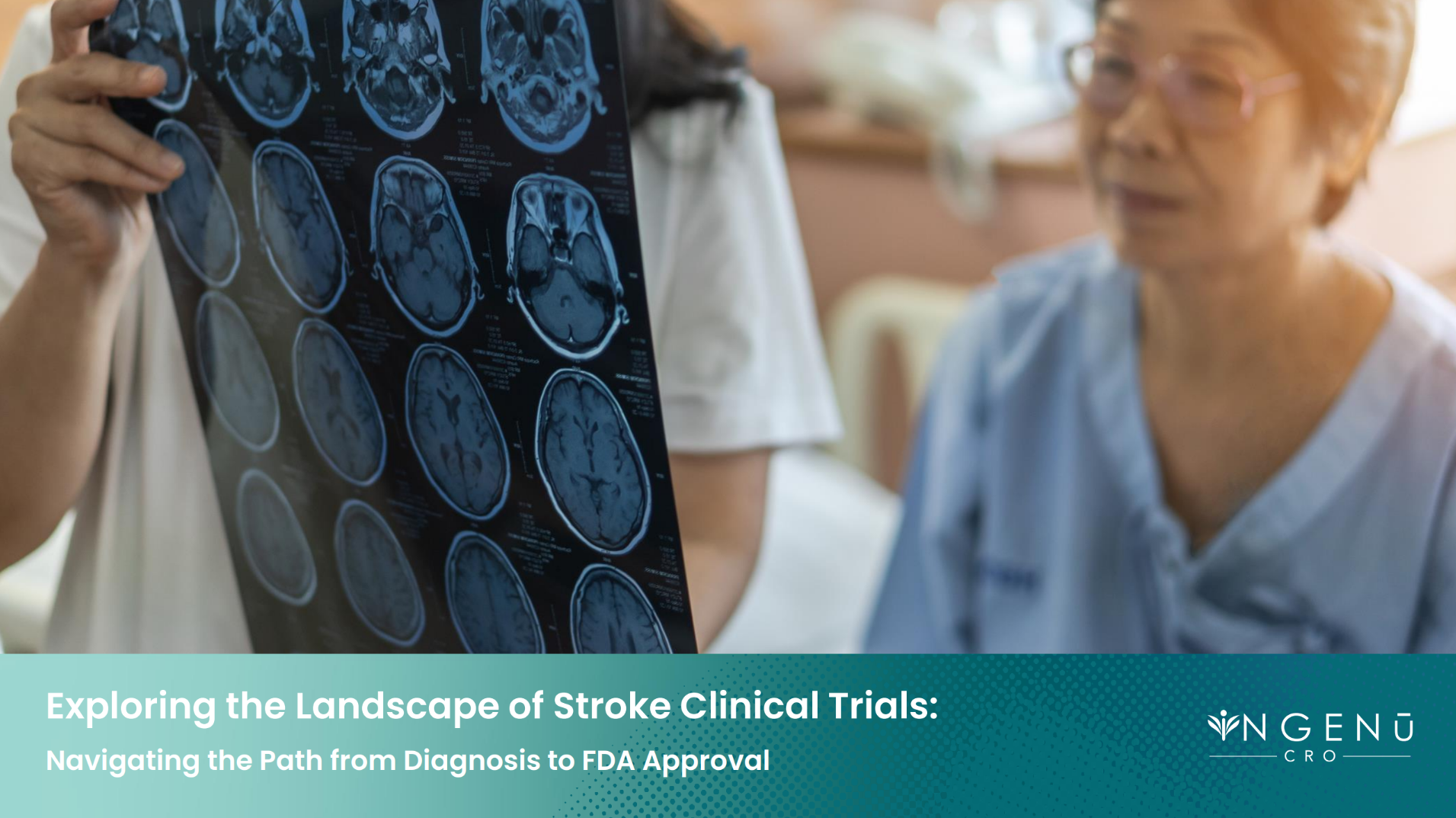
"Exploring the Landscape of Stroke Clinical Trials:
Navigating the Path from Diagnosis to FDA Approval"
What's Inside:
- Key FDA-Approved Pivotal Endpoints for Stroke Clinical Trials.
- Optimizing Recruitment with Adaptive Trial Designs for Stroke.
- Comprehensive Analysis of FDA-Approved Stroke Drugs.
- How Diagnostic Criteria for Stroke Have Evolved and Impact Research.
- Overcoming Common Pitfalls in Stroke Clinical Trials.
- And additional insights to drive your trial's success.
Access your complimentary whitepaper today:
Stroke
Stroke is a serious medical emergency that occurs when blood flow to a part of the brain is interrupted, either by a blockage (ischemic stroke) or by bleeding (hemorrhagic stroke).
This deprivation of oxygen and nutrients causes brain cells to die within minutes, often leading to permanent neurological damage or death if untreated. Stroke is a leading cause of long-term disability and the second leading cause of death globally. While ischemic stroke, caused by arterial blockages, is the most common type, hemorrhagic stroke results from ruptured blood vessels, and transient ischemic attacks (TIAs), or "mini-strokes," serve as critical warning signs for future major strokes.
Rapid intervention is essential to improve outcomes, reduce disability, and save lives, yet the path to effective treatment is fraught with challenges. This whitepaper examines the landscape of stroke clinical trials, covering diagnostic criteria, epidemiology, pivotal endpoints, and the complexities of endpoint determination.
At iNGENū, we are dedicated to advancing the understanding and treatment of Stroke. Through cutting-edge research and a patient-centered approach, our mission is to deliver innovative solutions that improve the lives of individuals and families impacted.
Stroke is the
3rd
most common cause of death in Australia
In 2023, 75% of strokes occurred in individuals over the age of
65
In 2023, 75% of stroked occurred in individuals over the age of
65
Our clinical team has over
120
years of combined clinical trial experience