Non-Clinical Services
iNGENū provides full end-to-end non-clinical research advisory services and can execute the standard FDA IND-enabling sequence of non-clinical studies.
Provision of
Comprehensive
Non-Clinical Services
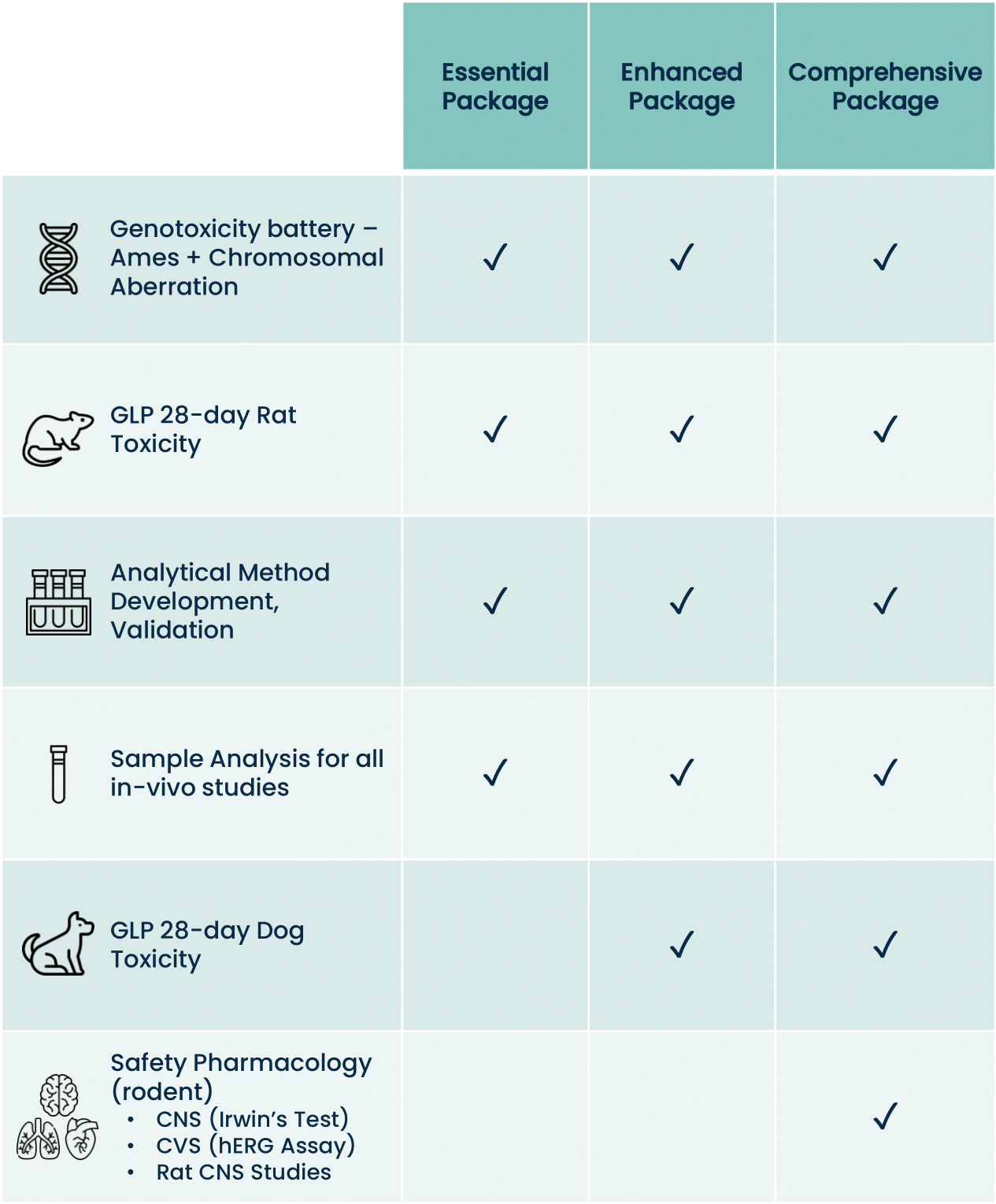
- Genetic Toxicology (“Genotox”) Battery (Ames Test, Chromosomal Aberration Assay, In Vitro Mouse Micronucleus, COMET study)
- Rodent and Second Species (dog, minipig, rabbit, NHP) GLP Toxicology for 4, 7, 28 days or chronic multi-dosing as specified by the FDA depending on the planned investigational product dosing strategy
- Safety Pharmacology Studies including Cardiovascular Telemetry Studies in dogs, Respiratory Studies (plethysmography in rats), and CNS Studies (modified Irwin test in rats)
- Bioanalytical Services needed (analytical method development, method validation, sample analysis)
- Full ADME Services (in vitro assays and PK in rodent, dog or minipig)
- Reproductive Toxicology
- Carcinogenicity Studies

Cost & Time Reduction
Our non-clinical research strategy advisory is primarily based on the M3(R2) FDA guidance for non-clinical safety studies.
For our clients seeking first-in-human clinical trials in Australia, a more abbreviated non-clinical study programme is usually possible.
Whilst due to local legislation, not all the in-life studies will be performed in Australia and can be performed at partner labs as per the multisite principles of GLP.
Non-clinical research (inclusive of both the in-life work and the bioanalytical work) is still eligible for the Australian Government 43.5% R&D Incentive in the same manner as clinical research.
This results in non-clinical research performed in Australia typically being 70-75% cheaper than similar studies being performed in the USA.
Additionally, the lag in North America to start second species (notably dog and NHP species) toxicology can be up to 9-12 months.
This level of delay is not being experienced in Asia-Pacific with animal studies being available within relatively short timeframes.
Our Preclinical Service Packages
Learn more about the full range of our Preclinical Service Packages by downloading our detailed PDF.
Simply fill out the form below to gain instant access.
Download Our Preclinical Service Packages
